Abstract
Background: In the era prior to the availability of novel agents, we have previously reported that the median progression-free survival (PFS) and overall survival (OS) of high-risk (HR) multiple myeloma (MM) patients (pts) undergoing a single autologous stem cell transplant (ASCT) was only 9.9 and 18.3 months, respectively (Chang H et al., Bone Marrow Transplant, 36 (2005), 793). The current study expands our experience in newly diagnosed HR pts and focuses on the outcomes in pts treated with single or tandem ASCT.
Methods: A retrospective chart review of myeloma pts was conducted based on pt information retrieved from the Princess Margaret Myeloma Database. 245 pts with HR MM diagnosed and treated in our centre from Oct 1998 to Dec 2016 were identified. Survival rates were calculated using the Kaplan-Meier product-limit method and the log rank-statistic was used for comparison of survival curves.
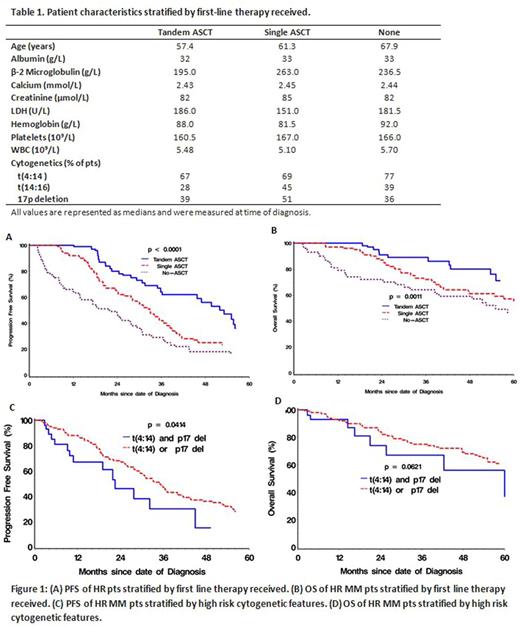
Results: Pts were considered HR based on the presence of any one of the cytogenetic features: t(4:14) (71%), t(14:16) (36%), and chromosome 17p deletion (42%). Characteristics at the time of MM diagnosis of the 245 pts identified as HR are summarized in Table 1. The median age was 60.9 years. First-line therapy included the following: tandem ASCT in 86 pts (35%), single ASCT in 84 pts (34%), and no ASCT in 75 pts (31%). Of the 170 pts who underwent single or tandem ASCT, 119 (70%) received maintenance therapy post-ASCT, including 84% of the tandem ASCT pts and 56% of the single ASCT pts.
Median follow-up was 29.3 months (range 1.1-123.7). The median PFS from date of diagnosis of all HR pts was 33.1 months [95% CI 29.3-37.0 months], while the median OS was 79.2 months [95% CI 58.3-90.9 months]. Both PFS (p= 0.0003) and OS (p= 0.0011) were significantly affected by first-line therapy with a median PFS of 53.1 vs 32.9 vs 21.8 months and median OS of 88.5 vs 71.9 vs 55.0 months in pts treated with tandem ASCT, single ASCT and no ASCT, respectively. Tandem ASCT pts had improved PFS and OS survival at 1, 2, and 5-years compared to single and non-transplant pts (Fig. 1A & B). Age at diagnosis significantly affected the PFS (p= 0.0003) and marginally affected the OS (p= 0.0748), likely due in part to our policy for the preferential use of fixed-duration non-ASCT as first-line therapy in older individuals (typically ≥70 years of age). On the other hand, serum beta 2- microglobulin levels correlated significantly with OS (p= 0.0023) while the association with PFS was not significant. Among ASCT pts, those who received tandem ASCTs vs single ASCT demonstrated a significantly better PFS (p= 0.0039) and showed a trend for improved OS (p= 0.0707). Amongst pts who received single ASCT, those with maintenance vs no maintenance demonstrated significantly better PFS (p=0.0082). Finally, pts with both t(4:14) and 17p deletion (28 pts) had a significantly decreased PFS (p= 0.0414) and a trend for decreased OS (p= 0.0621) compared to those with only one abnormality (Fig. 1C & D).
Conclusions: Although adverse factors such as older age and co-morbidities likely affected the ability to perform 1 or 2 autografts and likely introduced bias into our analysis, our data nevertheless suggest that tandem ASCT is an effective approach in extending PFS and OS among HR MM pts when compared to single transplants or none at all in the real-world setting. Multivariate analysis is underway to further delineate the factors contributing to PFS and OS in HR pts. As in other transplant studies, the PFS is improved in pts who receive maintenance therapy after single ASCT. Efforts should be made to offer newly diagnosed HR MM pts a program that includes tandem transplantation, if feasible, as well as maintenance therapy post-transplant. Pts with "ultra HR" MM, i.e., those with both t(4;14) and 17p deletion, require new innovative approaches.
Reece: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Paid expert testimony, Research Funding, Speakers Bureau; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Other: Paid expert testimony, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Otsuka: Research Funding. Chen: Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Abbvie: Honoraria; Amgen: Honoraria. Kukreti: Celgene: Honoraria; Amgen: Honoraria. Prica: Janssen: Honoraria; Celgene: Honoraria. Tiedemann: BMS Canada: Honoraria; Celgene: Honoraria; Takeda Oncology: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Novartis: Honoraria. Trudel: Janssen: Research Funding; Amgen: Consultancy, Honoraria; Astellas: Research Funding; Celgene: Consultancy, Honoraria; Takeda: Honoraria; GlaxoSmithKline: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal